
I’m a Soil Nitrogen Researcher.
 The above statement is something I sometimes find hard to believe. You see, I failed maths in high school, and had no interest in science whatsoever, until fairly recently. After leaving high school, music was all that was on my mind. It led me to the US and back, and I’m grateful for it.
The above statement is something I sometimes find hard to believe. You see, I failed maths in high school, and had no interest in science whatsoever, until fairly recently. After leaving high school, music was all that was on my mind. It led me to the US and back, and I’m grateful for it.
But after burning out on music-making, and deciding it was something I shouldn’t do full-time, I wanted to do something ‘worthwhile’ and ‘meaningful’. So, I went back to university to study science. It has been one of the hardest things I’ve ever committed to, but with the help and support of family, and with a bit of grit and determination, I clawed my way through my undergraduate degree, and ultimately a PhD!
Now, I’m a postdoctoral researcher at the Swedish University of Agricultural Sciences, in Umeå, Sweden. I never thought I’d be saying that!
My Research
My research focuses on an emerging soil sampling technology called microdialysis. This technology uses tiny probes to sample solutes in soil with minimal disturbance to soil structure. Understanding what happens to solutes in undisturbed soil environments is crucial to many functions of soil ecology, including microbial and plant utilisation of available nitrogen – a nutrient vital to all life.
I hope to develop microdialysis further by investigating several aspects of the nitrogen cycling using the method, identifying strengths and weaknesses along the way. I hope that my research will help develop a more accurate view of nitrogen cycling and microbial function in soil undisturbed by experimental factors. Read more about my research…
Publications
Buckley, S., Brackin, R., Näsholm, T., Schmidt, S., Jämtgård, S. 2022. The influence of sucrose on soil nitrogen availability – A root exudate simulation using microdialysis. Geoderma, 409, 115645. (Link)
Plett, K.L., Buckley, S., Plett, J.M., Anderson, I.C., Lundberg-Felten., J., Jämtgård, S. 2021. Novel Microdialysis Technique Reveals a Dramatic Shift in Metabolite Secretion during the Early Stages of the Interaction between the Ectomycorrhizal Fungus Pisolithus microcarpus and its host Eucalyptus grandis. 2021. Microorganisms, 9, 1817. (Link)
Westermann, M., Brackin, R., Robinson, N., Salazar Cajas, M., Buckley, S., Bailey, T., Redding, M., Kochanek, J., Hill, J., Guillou, S., Freitas, J.C.M., Jr., Wang, W., Pratt, C., Fujinuma, R., Schmidt, S. 2021. Organic Wastes Amended with Sorbents Reduce N2O Emissions from Sugarcane Cropping. Environments 2021, 8, 78. (Link)
Buckley, S., Brackin, R., Jämtgård, S., Näsholm, T., Schmidt, S., 2020. Microdialysis in soil environments: Current practice and future perspectives. 2020. Soil Biology and Biochemistry 143, 107743. (Link)
Buckley, S., Allen, D., Brackin, R., Jämtgård, S., Näsholm, T., Schmidt, S., 2019. Microdialysis as an in situ technique for sampling soil enzymes. Soil Biology and Biochemistry 135, 20-27. (Link)
Jones, A.R., Gupta, V.V.S.R., Buckley, S., Brackin, R., Schmidt, S., Dalal, R.C., 2019. Drying and rewetting effects on organic matter mineralisation of contrasting soils after 36 years of storage. Geoderma 342, 12-19.
Brackin, R., Buckley, S., Pirie, R., Visser, F., 2019. Predicting nitrogen mineralisation in Australian irrigated cotton cropping systems. Soil Research 57, 247-256.
Buckley, S., Brackin, R., Näsholm, T., Schmidt, S., Jämtgård, S., 2017. Improving in situ recovery of soil nitrogen using the microdialysis technique. Soil Biology and Biochemistry 114, 93-103. (Link)
Buckley, S., Brackin, R., Schmidt, S., 2016. Microdialysis – a sensitive method for estimating plant-available N released during litter decomposition, 7th International Nitrogen Initiative Conference, Melbourne, Australia. (Link)
Brackin, R., Schmidt, S., Walter, D., Bhuiyan, S., Buckley, S., Anderson, J., 2017. Soil biological health—what is it and how can we improve it?, Australian Society of Sugar Cane Technologists, pp. 141-154. (Link)
Brackin, R., Nasholm, T., Robinson, N., Guillou, S., Vinall, K., Buckley, S., Lakshmanan, P., Schmidt, S., Inselsbacher, E., 2016. Microdialysis–a new technology for investigating soil nitrogen fluxes in the rhizosphere, 7th International Nitrogen Initiative Conference, Melbourne, Australia. (Link)
Awards
- – Sugar Research Australia PhD Top-Up Scholarship (2015)
- – Australian Postgraduate Award (APA, 2015)
My Research: Soil Nitrogen Availability
Nitrogen (N) is one of the most vital nutrients on the planet. All life needs to grow and function, and we spend a lot of our time trying to get enough of it – whether we know it or not.
Plants need N too; a fact farmers know all too well. It’s so valuable to crop production, we use 2% of the world’s energy to make enough N fertilisers to feed crops around the world.But more often than not, we apply too much N to farms. Crops can only acquire so much N – the rest can be leached into waterways and oceans, with terrible consequences for affected ecosystems; alternatively, N can be lost into the atmosphere as potent greenhouse gases nearly 300-times more potent that carbon dioxide. We need to get better at using N in our cropping systems, and quickly.
MEASURING SOIL NITROGEN
The soil N cycle is a notoriously complex series of transformations and pathways, but knowing how soil N behaves and transforms over time is incredibly important for tailoring our inputs so losses are minimised, and uptake by crops is as efficient as possible.
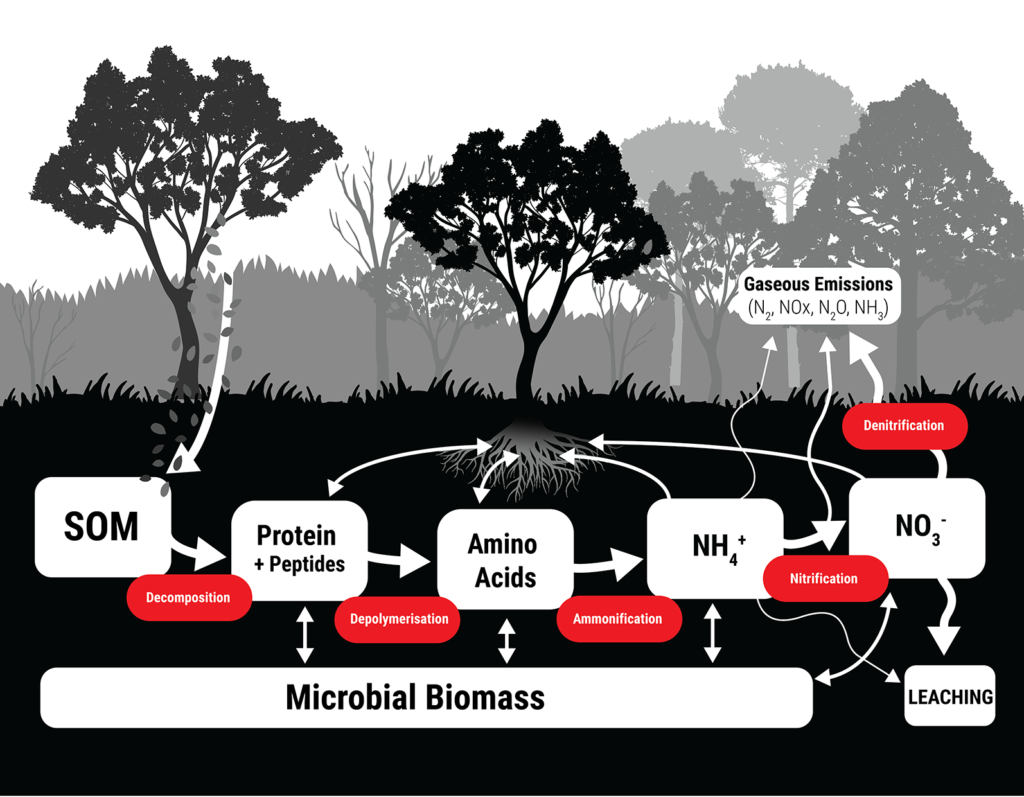
The nitrogen cycle in a natural ecosystem. Soil organic matter (SOM) releases organic nitrogen forms like proteins, peptides and amino acids through decomposition. Further transformations by microbes release inorganic N like ammonium (NH4+) and nitrate (NO3-), both of which can be lost from soil through leaching, or through gaseous emissions into the atmosphere.
But if we want to intimately understand the N cycle, we need methods of measuring N which are sensitive enough to discriminate between these complex transformation pathways. Better yet, we need methods that can measure how much, and what kinds of N are truly available to plant roots.
We have plenty of long-standing methods of measuring N in soil samples – many of these methods are cheap and quick to implement. However, most have drawbacks – most notably, they dramatically disturb the soil environment, leading to large, unwanted artefacts in our measurements; meaning poor estimations of N in our samples, and a poor representation of what kinds of N plants are presented with in an undisturbed soil.
SOIL MICRODIALYSIS
With my research, I’m helping to pioneer a new sampling technique called microdialysis (below) which uses tiny probes to collect soil N samples from within a soil profile without the need to dig it up and destroy it with sieving and shaking. So far, I’ve found that microdialysis can show greater sensitivity to changes in N availability than conventional sampling methods (link), meaning we might be missing a large part of the soil N story by simply choosing to use conventional methods.

The microdialysis technique, being used to sample nitrogen in a Swedish forest soil during a research trip in 2016.
We’ve also found ways to improve the sensitivity of microdialysis, by modifying the length of the probes (link). This means big improvements to the level of accuracy and precision in determining N fluxes in complex soil environments, as well as improving the recovery of other low-concentration solutes from soil.
It is my opinion that microdialysis will help provide a clearer understanding of the amount and types of N available to plants and microbes, and in turn help us develop better ways, and better timing of nutrient delivery to the food crops that minimises losses, but maintains crop production.
